Lithium cobalt oxide (LiCoO2, LCO) cathodes remain the dominant cathode material for lithium-ion batteries in consumer electronics. However, when operated at elevated voltages (>4.5 V), layered LCO cathodes face severe challenges, including irreversible structural phase transitions, pronounced interfacial side reactions, surface transition-metal dissolution, and lattice-oxygen participation in electrochemical processes. These issues lead to an increased cathode–electrolyte interphase (CEI) impedance and rapid capacity decay. To address these challenges, Professor Feng Pan’s research group previously developed strategies such as surface coating regulation and fluorinated electrolyte design. These approaches enabled the precise construction of functional CEI layers on high-voltage LCO surfaces, significantly enhancing the interfacial structural stability and reversibility of bulk phase transitions (Adv. Energy Mater., 2024, 14, 2402223; Adv. Mater., 2024, 36, 2408875; Energy Environ. Sci., 2024, 17, 7944; Adv. Funct. Mater., 2025, 2504165).
Nevertheless, under elevated-temperature conditions, interfacial side reactions between cathodes and electrolytes are further aggravated, accompanied by significant hydrofluoric acid generation. This severely corrodes both CEI films and cathode materials, ultimately resulting in CEI breakdown and interfacial instability. Hence, there is an urgent need for a compatible interfacial regulation strategy that can in situ construct dense and stable passivation layers to effectively suppress electrolyte decomposition and irreversible cathode degradation under high-voltage and high-temperature conditions.
Recently, leveraging a materials genome approach—focused on three key aspects: functional building blocks, their ordering, and their interactions—Pan’s team successfully predicted electrolyte systems capable of enhancing the interfacial stability of LCO cathodes under high-voltage operation. By introducing triethyl phosphate (TEP) and 1,3-propane sultone (PS) into conventional carbonate-based electrolytes, the researchers enabled the in-situ construction of a three-layer functional CEI film on the LCO surface: The innermost Li3PO4 layer suppresses lattice-oxygen activity and improves ionic conductivity; The intermediate LiF framework layer blocks electron transport; The outermost Li2SO3 layer provides excellent thermal stability and adhesion. The synergistic effect of these components results in a compact and durable interfacial passivation layer that remains stable under harsh high-temperature and high-voltage conditions. This CEI design strategy exhibits strong generality and scalability, holding promise for broader applications in other layered oxide cathode materials for lithium batteries. The related research has been published in National Science Review under the title “Engineering durable interphases for high-voltage Li-ion batteries under thermal stress” (National Science Review, 2025, nwaf345).
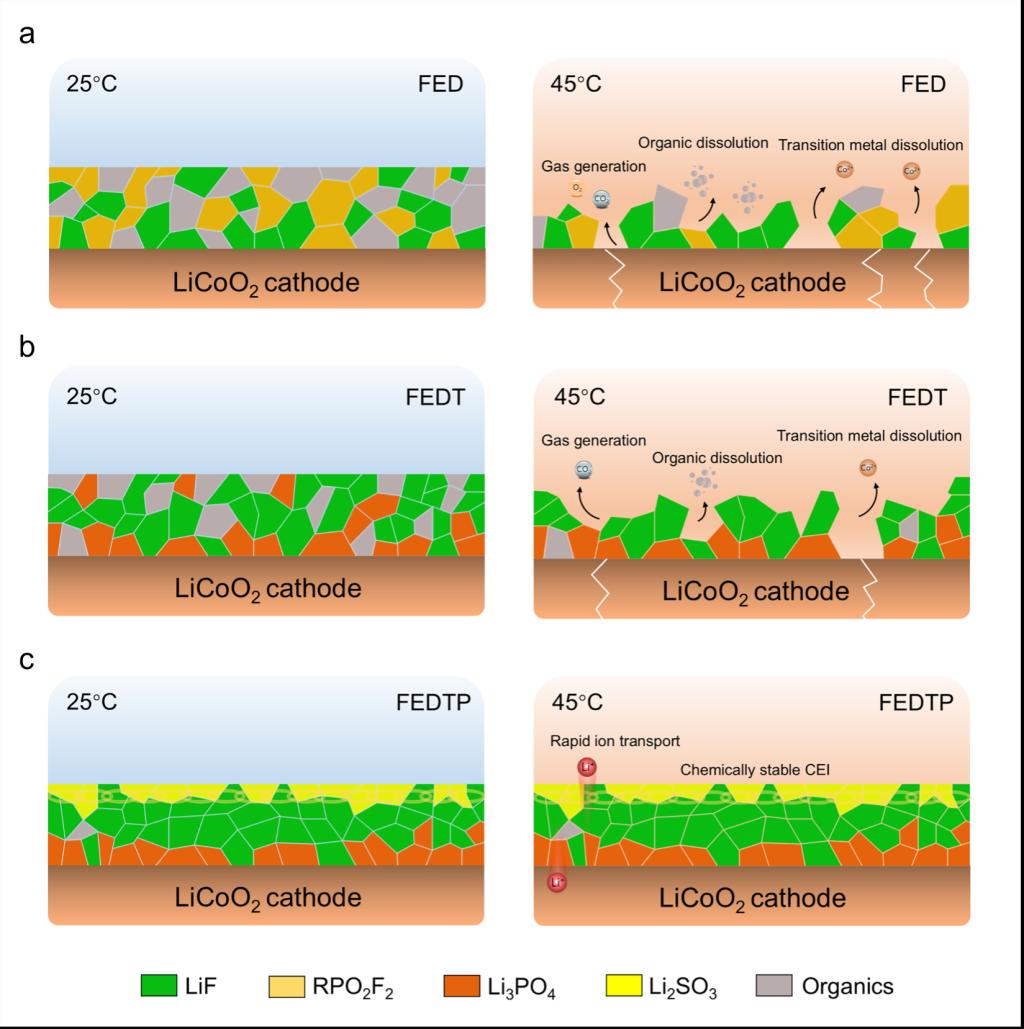
Figure 1. Schematic illustration of the electrolyte regulation mechanism at 25 °C and 45 °C
This work was carried out under the joint supervision of Professor Feng Pan and Associate Researcher Luyi Yang from the SAM, Peking University Shenzhen Graduate School, together with Dr. Guorui Zheng from Tsinghua University. Dr. Shiming Chen, a PhD graduate of PKUSZ and currently a postdoctoral fellow in the Department of Chemistry at The University of Hong Kong, and Wenguang Zhao, a PhD candidate at PKUSZ, are co-first authors of the paper. The research was supported by the National Natural Science Foundation of China, the International Research Center for Electric Vehicle Power Batteries and Materials, the Guangdong Provincial Key Laboratory of New Energy Materials Design and Computation, and the Shenzhen Key Laboratory of New Energy Materials Genome Preparation and Testing.
Link to the paper: https://doi.org/10.1093/nsr/nwaf345
