With the rapid growth of the consumer electronics industry, layered lithium cobalt oxide (LiCoO2, LCO) cathode materials are facing increasingly stringent demands for higher energy density and longer cycle life. However, at voltages above 4.5 V (vs. Li+/Li), LCO undergoes severe structural degradation, leading to rapid capacity fading. Conventional polyvinylidene fluoride (PVDF) binders, while exhibiting excellent electrochemical stability, require the use of toxic solvent N-methyl-2-pyrrolidone (NMP) during electrode fabrication and show relatively weak interactions with cathode particles, making them ineffective in suppressing interfacial side reactions. As a result, developing a low-cost, environmentally friendly binder suitable for high-voltage LCO cathodes has become a pressing challenge.
Sodium carboxymethyl cellulose (CMC), widely used as an aqueous binder for anode materials, has rarely been reported in high-voltage cathode applications. Through a series of electrochemical tests, the research team found that CMC undergoes severe decomposition under high-voltage and high-current conditions, triggering extensive interfacial side reactions. These reactions cause irreversible phase transitions of the cathode material and the dissolution of transition-metal ions, ultimately compromising battery performance.
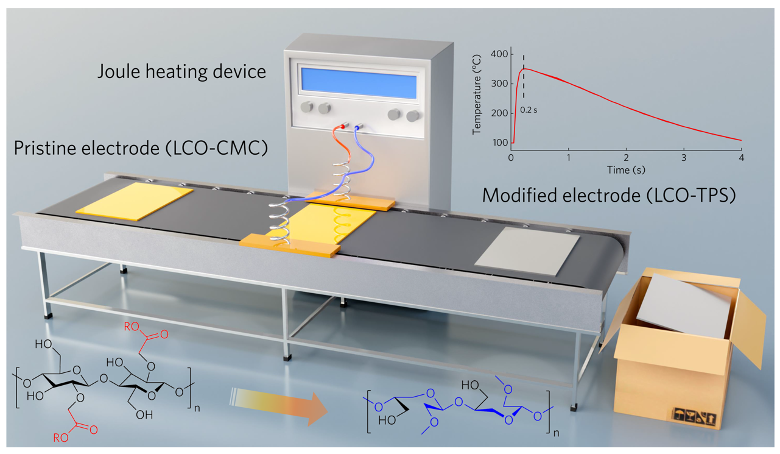
Figure 1. Schematic illustration of the synthesis of the CMC-TPS binder
To address the above challenges, the research team proposed a modification strategy based on ultra-fast thermal pulse sintering (TPS). By applying rapid heating and cooling, the molecular structure of CMC was tailored: unstable carboxyl functional groups under high voltage were removed, while ether-rich chain structures were formed (denoted as CMC-TPS). This structural transformation significantly enhanced Li⁺ transport kinetics during cycling. Meanwhile, localized high temperatures induced partial carbonization of the binder, improving the overall conductivity of the electrode. Further investigations, combining multidimensional characterization techniques, revealed that thermal treatment enabled the CMC-TPS binder to form a uniform coating layer on the cathode particle surfaces. This coating effectively suppressed irreversible phase transitions and interfacial side reactions, thereby markedly improving the electrochemical performance of LCO. Additionally, during thermal treatment, the carboxyl groups of the binder underwent dehydration-condensation reactions with hydroxyl groups on the current collector, forming covalent bonds that strengthened electrode adhesion. Theoretical calculations further demonstrated that the CMC-TPS binder lowers the O 2p band center energy on the LCO surface, stabilizes the cathode lattice under high-voltage conditions, and suppresses transition-metal dissolution, thus significantly enhancing interfacial stability.
This study represents the first successful design and synthesis of a multifunctional aqueous binder for LCO cathodes based on theoretical calculations. The strategy is also applicable to graphite anodes, offering a new pathway for the development of high-voltage LCO-based lithium-ion batteries. The related research has been published in Angewandte Chemie International Edition under the title “Tailoring Sodium Carboxymethylcellulose Binders for High-Voltage LiCoO2 via Thermal Pulse Sintering” (DOI: 10.1002/anie.202423796).
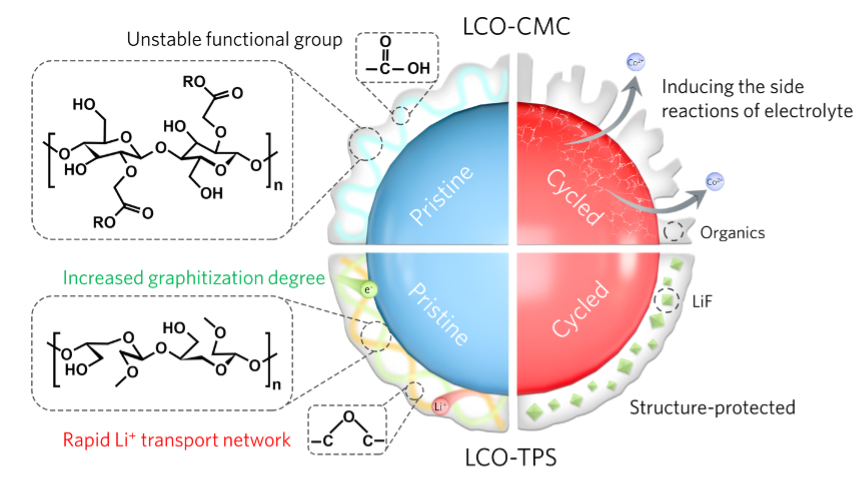
Figure 2. Mechanism of CMC-TPS binder in improving the performance of LCO cathodes
This work was jointly supervised by Professor Feng Pan and Associate Researcher Luyi Yang from Peking University, and Assistant Professor Zuwei Yin from Xiamen University. Shiming Chen, a PhD candidate at the SAM, Peking University Shenzhen Graduate School, and Hengyao Zhu, a PhD candidate in the Department of Chemistry, College of Science, City University of Hong Kong, are co-first authors. The research was supported by the National Natural Science Foundation of China, the International Research Center for Electric Vehicle Power Batteries and Materials, the Guangdong Provincial Key Laboratory of New Energy Materials Design and Computation, and the Shenzhen Key Laboratory of New Energy Materials Genome Preparation and Testing.
