Constructing a stable cathode electrolyte interphase (CEI) is a critical technological pathway for achieving performance breakthroughs in high-voltage lithium-ion batteries. However, the formation of CEI involves complex, irreversible electrochemical reactions between the electrolyte and cathode materials, and its specific formation mechanisms remain poorly understood. Existing studies indicate that anions play a dominant role in CEI formation, primarily due to their tendency to adsorb and decompose more readily than carbonate solvents at the “inner-Helmholtz plane” (IHP) of the cathode material surface. Nevertheless, the academic community still lacks in-depth exploration of anion adsorption behavior and its specific role in CEI formation, which limits further improvements in the stability of high-voltage lithium-ion batteries.
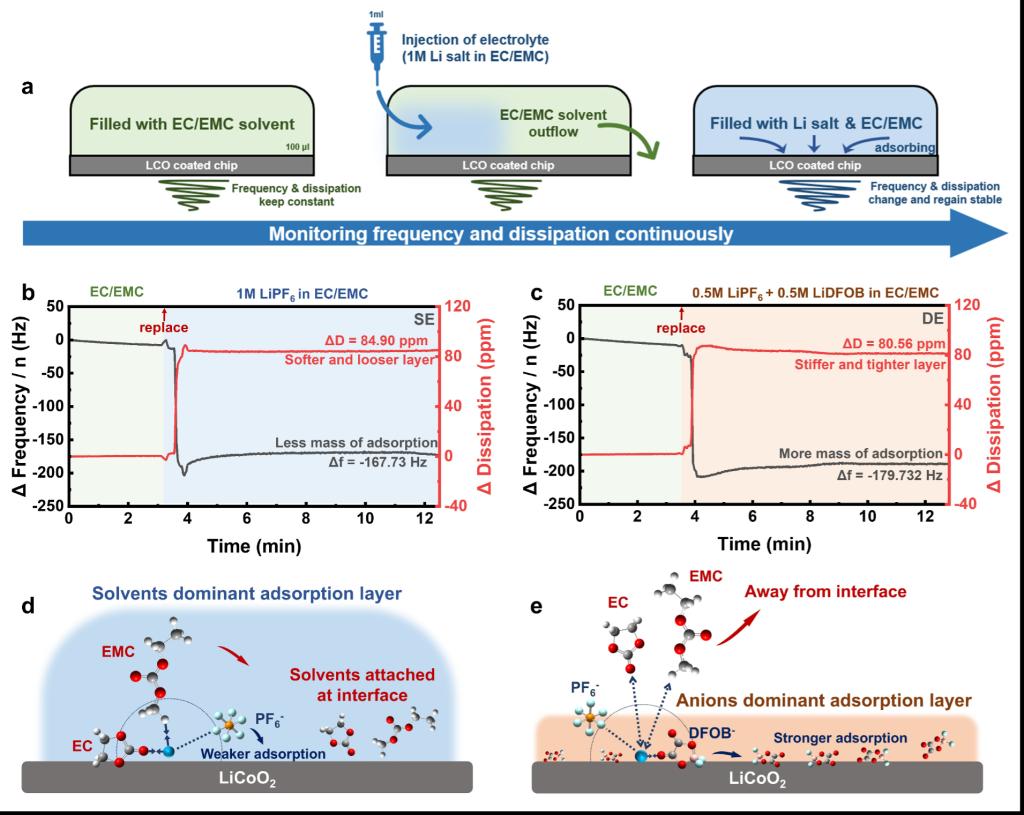
Figure 1: Anion Adsorption Behavior and Interface Structure Evolution
To address these challenges, the research team employed multiple in-situ interface characterization techniques to systematically reveal, for the first time, the adsorption behavior of anions at the IHP and their role in CEI formation. The study found that the adsorption behavior of different anions on the LiCoO2 (LCO) surface directly determines the chemical composition and structural stability of the CEI. Using quartz crystal microbalance with dissipation monitoring (QCM-D) experiments, the team quantitatively compared the adsorption behaviors of different anions on the LCO surface. Combined with advanced characterization techniques such as in-situ attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) and in-situ confocal Raman spectroscopy, they thoroughly analyzed how different anion adsorption behaviors regulate the CEI formation process. The results demonstrated that, compared to conventional PF6⁻, DFOB⁻ anions exhibit higher adsorption energy on the LCO surface, forming an anion-dominated adsorption layer at the IHP. This layer effectively blocks direct contact between organic solvent molecules and the cathode surface, significantly suppressing the decomposition reactions of carbonate solvents during CEI formation. This mechanism ultimately promotes the formation of a denser, inorganic-rich CEI film, enabling the LCO cathode to exhibit excellent cycling stability under ultra-high voltage conditions (>4.7V).
This study not only deepens the academic understanding of CEI formation mechanisms but also provides critical theoretical guidance for the future design of high-performance lithium-ion battery electrolyte systems and the optimization of electrode/electrolyte interfaces. The findings were published in the internationally renowned chemistry journal Angewandte Chemie International Edition under the title “Anion Adsorption at the Inner-Helmholtz Plane Directs Cathode Electrolyte Interphase Formation” (DOI: 10.1002/anie.202425535).

Figure 2: Paper Published in Angew. Chem. Int. Ed.
The work was completed under the joint guidance of Professor Feng Pan from Peking University, Associate Researcher Yang Luyi, and Dr. Kang Xu, Chief Technology Officer of SES AI. Dr. Yuchen Ji, a PhD graduate, and Yuxiang Huang, a master’s graduate from the SAM at Peking University’s Shenzhen Graduate School, were co-first authors of the paper. The research was supported by the National Natural Science Foundation of China, the International Joint Research Center for Power Batteries and Materials for Electric Vehicles, the Guangdong Key Laboratory for New Energy Materials Design and Computation, and the Shenzhen Key Laboratory for New Energy Materials Genome Preparation and Testing.
